Here’s what we can learn from the bacteria in the clam that sank a thousand ships

They stranded Christopher Columbus in Jamaica. They brought down the Spanish Armada. They sent San Francisco’s piers crumbling into the sea.
For as long as humanity has been putting wooden structures into the ocean, shipworms have been laying waste to them.
“There are reports going back to the ancient Greeks and Romans, complaining about shipworms destroying their docks and their boats,” says Dan Distel, who directs Northeastern’s Ocean Genome Legacy Center. “During the age of wooden boats, it was a huge challenge.”

Dan Distel directors Northeastern’s Ocean Genome Legacy Center. Photo by Matthew Modoono/Northeastern University
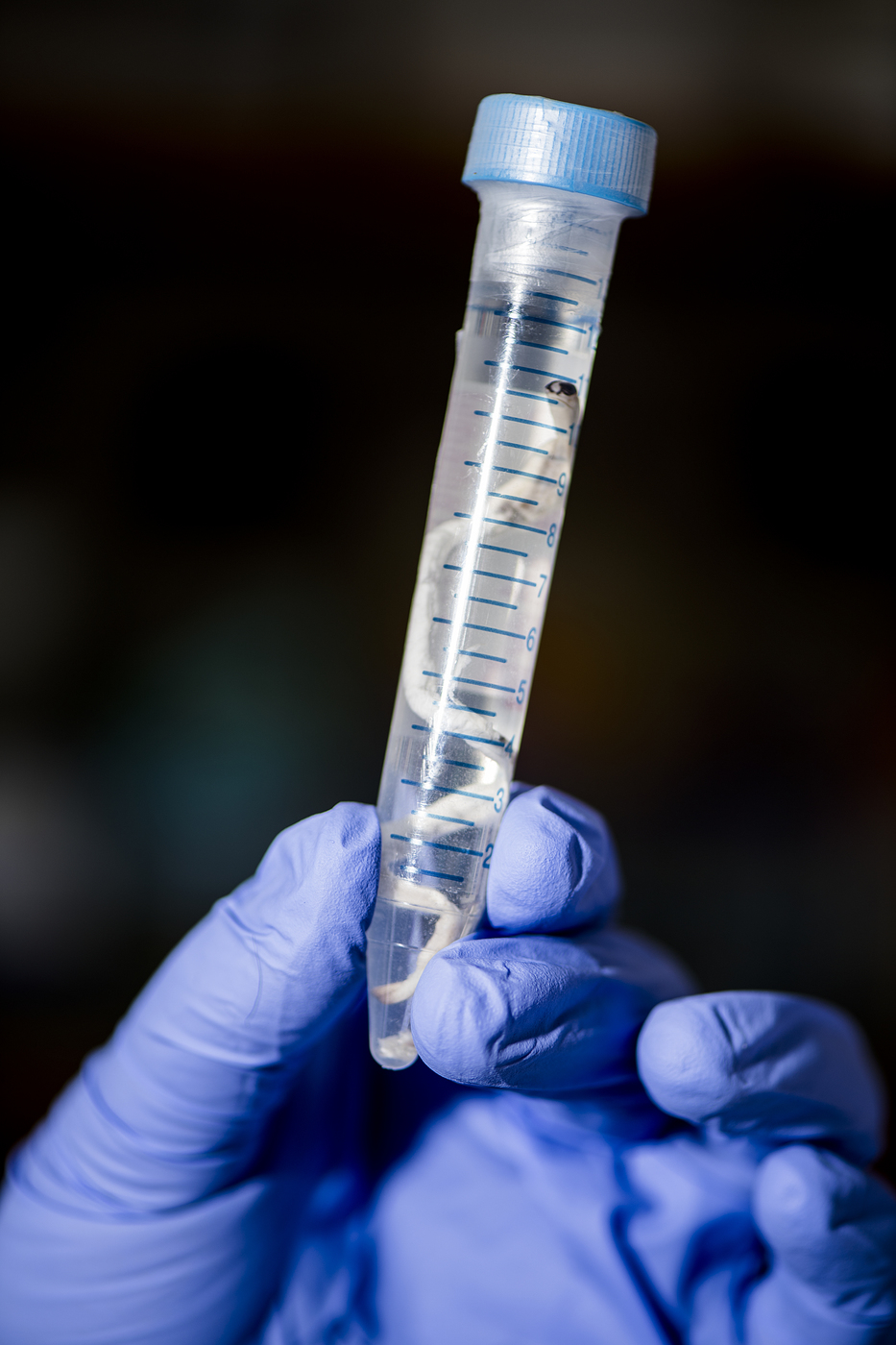
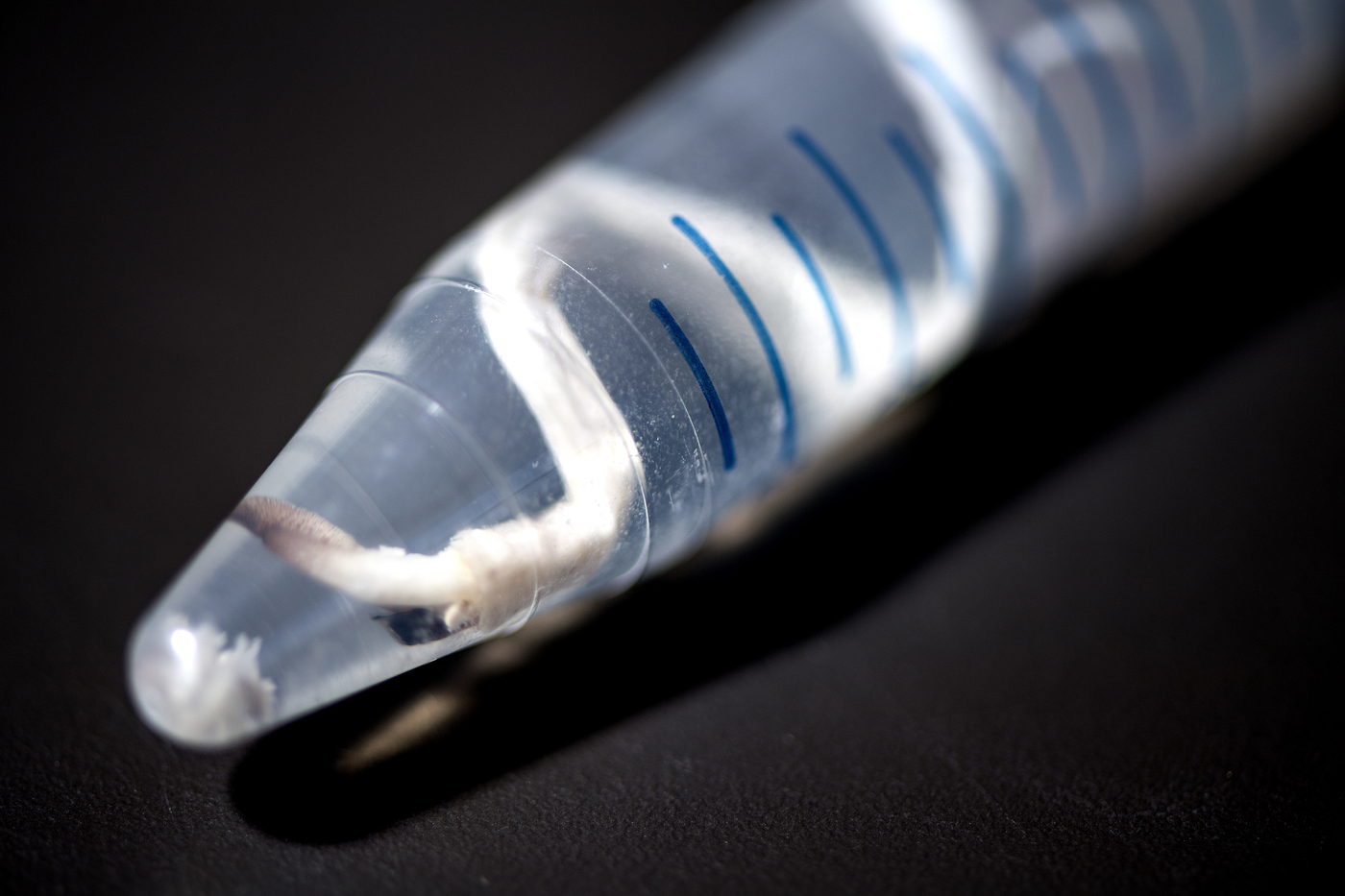
Shipworms are long, thin mollusks famed (and feared) for their ability to eat wood. But they can’t do it alone. They rely on bacterial partners to break the wood down into nutrients they can use. Studying these bacteria could reveal more efficient ways to use the wood and plant waste we generate here on land.
In a paper released earlier this year, Distel and his colleagues described one of these bacteria, naming it Teredinibacter waterburyi.
“It’s a long, hard road to name bacteria,” says Distel, who is working on naming several other species his team has grown. “But nobody takes any bacteria seriously unless they have a name.”
Teredinibacter waterburyi is only the second named species of wood-eating shipworm bacteria. It creates enzymes to help its host, the feathery shipworm, which is found along the Pacific coast of North America, break down cellulose in wood.
But the bacteria don’t reside in the gut, where digestion takes place. Teredinibacter waterburyi and several other bacterial partners live inside the cells of the animal’s gills. The enzymes they manufacture must get out of the bacterial cell, across the animal cell’s cytoplasm, through another cell wall, and then, all the way to the gut. (Distel is still figuring out exactly how this works.)
Those enzymes are one of the reasons researchers are so interested in shipworm microbes. They can break down the complex material in the cell walls of plants, lignocellulose, into sugars.

“Lignocellulose is the most abundant biological material on earth,” Distel says. Discarded plant matter, such as wood, corn and wheat stalks, and paper waste, could be converted into sugars and used to make biofuels, plastics, or other resources.
“Learning how these bacteria do it can provide us clues as to how to more efficiently convert waste, domestic waste, agricultural waste, industrial waste, forest product waste, into things that can be used for fuels or various chemicals,” Distel says.
These bacteria could also help researchers learn more about how harmful bacteria infect us. While Teredinibacter waterburyi doesn’t harm the shipworm it lives in, it still has to get inside the shipworm’s cells.
“Understanding how a bacterium infects a host cell is a question that’s really important,” Distel says. “There’s likely very similar mechanisms between pathogens infecting hosts and [helpful bacteria] infecting hosts.”
And if those weren’t enough potential contributions from a single-celled organism, Teredinibacter waterburyi could also be a rich source of compounds that could be used as drugs and medicines.
Bacteria produce molecules that can manipulate the behavior or biology of the cells around them, called secondary metabolites. Some of these have the potential to be developed into drugs to kill cancer cells or treat infections. Already, two antibiotics have been discovered in shipworm bacteria, Distel says, and one of them is now being investigated as an anti-parasite drug.
“This genus, Teredinibacter, turns out to have a lot of genes for secondary metabolism—a lot of genes for making things that could potentially be antimicrobial; antibiotic; neuroactive, that affect the neurological system of other organisms; or cytotoxic, things that kill other cells,” Distel says. “There are good drug lead compounds coming out of this.”
For media inquiries, please contact Shannon Nargi at s.nargi@northeastern.edu or 617-373-5718.





