How a Northeastern scientist is turning carbon dioxide into renewable ethanol
Assistant professor Magda Barecka is designing a chemical reactor that could produce renewable, carbon-neutral ethanol for use in fuels. Her research recently received support from the U.S. Department of Energy.
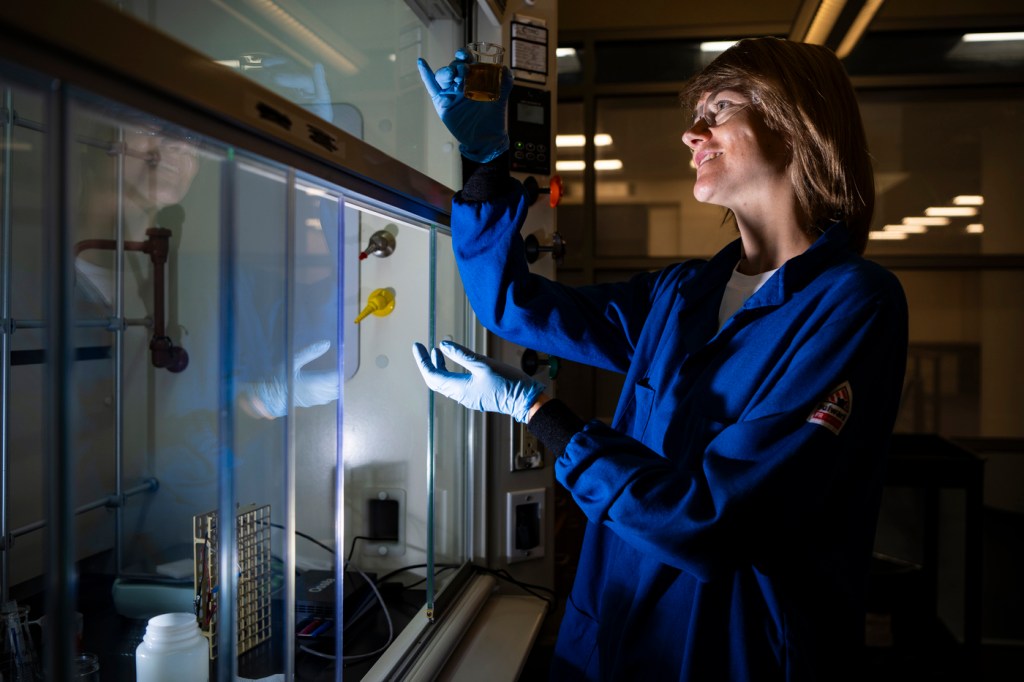
Magda Barecka is envisioning a world without fossil fuels.
An assistant professor of chemical engineering and chemistry and chemical biology at Northeastern University, Barecka says that the aim of her research is to enable “our society to function, to have all the chemicals that we need, without using fossil fuels.”
Like alchemists, Barecka and her team transform one thing into another — but instead of lead into gold, they transform carbon dioxide into ethanol.
After extracting carbon dioxide from the atmosphere and introducing it to a proprietary liquid in the base of their electrochemical reactor, a series of electrified metal plates do the transformative work, producing liquid ethanol that can be synthesized into a variety of fuels.
Despite its ability to burn — and thus serve as a fuel — ethanol is an alcohol and not a fossil fuel.
When it’s eventually burned, the ethanol will release carbon dioxide back into the atmosphere — but because that CO2 was originally taken from the air, the result is a carbon-neutral cycle.
Barecka and her team have published extensively on these topics, including here and here.
Their chemical reactor does require electricity, however, which still often comes from fossil fuel-based power plants.
Featured Posts
If the electricity required to make this conversion were to come from a renewable source, then the entire process could be made carbon neutral, a closed loop.
Now, Barecka’s research has received support from the U.S. Department of Energy through a program that supports projects that use “renewable energy sources like wind and solar to produce liquids for sustainable fuels or chemicals that can be transported and stored as easily as carbon-intensive liquids like gasoline or oil.”
“It takes a relatively long time to connect a new, renewable power plant to the grid,” Barecka says, which “could deliver energy, renewable energy, [with a] very low carbon footprint,” and they come with their own logistical and financial complications.
Working with the Department of Energy, Barecka and her team are “looking into what is the most efficient way to use this renewable electricity. So we really care about the amount of the product that we can get by using a specific amount of electricity coming from a renewable plant.”
Using electricity from a renewable power plant “to drive any chemical reactions,” she says, “we could really overcome the main bottleneck that we see right now in the construction of new renewable power plants.
“There’s a very interesting synergy, that by providing a technology that uses renewable electricity, we can ultimately help to have more renewable power plants in general.
“We want to create something that will unlock many different possibilities, that you can get many things that our society needs” without putting more greenhouse gases into the air, Barecka says.
Jet fuel is just one of the applications ethanol can be employed for.
The aviation market, Barecka continues, “is very customer oriented, and they take very seriously the fact that people are starting to be very careful about the carbon footprint of their travels.”
The aviation market already has “an existing method to synthesize fuels that use ethanol,” she says. “So that’s a great thing that we’re doing, really plugging into something existing so it can have a broader impact.”
“Whatever we develop, we want it to be applicable in the real-life scenario,” she says. “So that means that it has to fit into an existing supply chain.”
“I do recognize that there are a lot of logistics and multiple factors beyond science that are involved” in shifting away from fossil fuel reliance, Barecka says. “But I do believe that if we think deeply and differently about how we perform chemical reactions, we do have opportunities to use different energy sources.
“We can use those energy sources efficiently, and there is nothing from a thermodynamic point of view that prevents us from using different carbon sources than fossil fuels.”











