What a tiny, 3D gut can tell us about gastrointestinal disorders
Northeastern University chemical engineering professors Abigail and Ryan Koppes built a model that combines synthetic polymers and live tissue, mimicking how vital neurons operate within the small intestine.
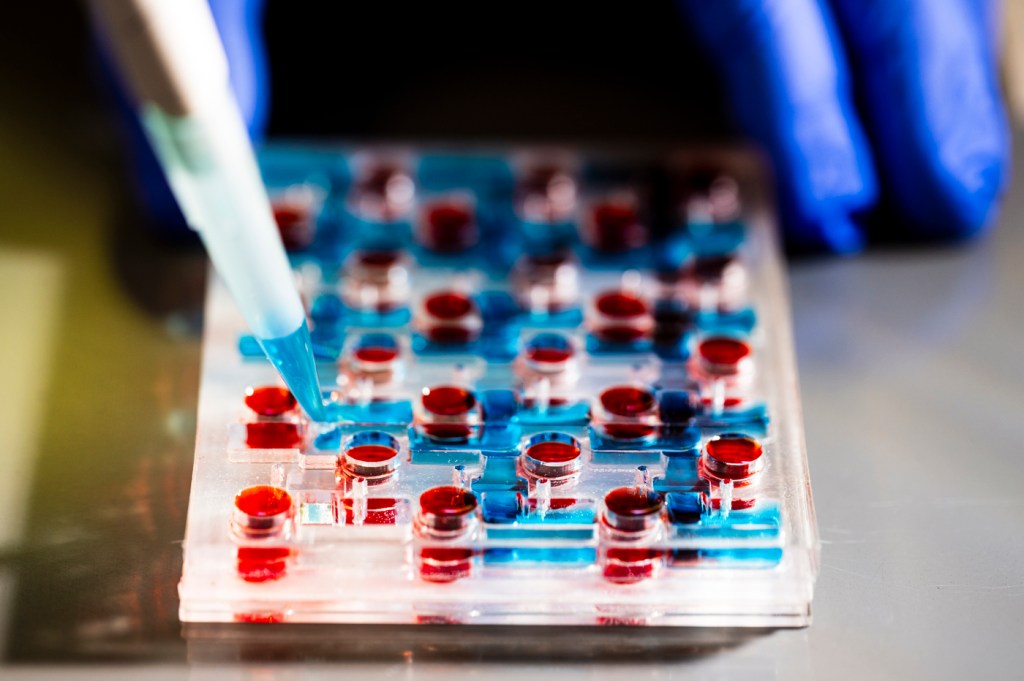
The relationship between our nervous and digestive systems is a relatively new area of scientific study. But what Northeastern University researcher Abigail Koppes calls the “brain gut” connection has vast implications for our overall health that we’re just beginning to understand.
“It’s a complex network,” says Koppes, an associate professor of chemical engineering. “We know, for example, that people who have autism, Parkinson’s or Alzheimer’s disease often have bowel dysfunction. We know that people who have Crohn’s disease, ulcerative colitis or IBS [irritable bowel syndrome] often co-present with anxiety or depression. Until about 2018, no one had really proven these connections existed.”
In their lab, Koppes and fellow associate chemical engineering professor Ryan Koppes (her husband) are building tiny, structural models that will help elucidate those connections. In September, the Koppeses, along with PhD students Kyla Kaiser and Jessica Snyder (both now graduates), published a paper in the academic journal Advanced Functional Materials outlining the construction of a 3D model that simulates interactions in the human small intestine. Specifically, their model aims for a closer look at enteric neurons: nerve cells that keep things moving smoothly within the intestinal tract.

The Koppes model, a dozen of which fit on a plastic platform the size of a postcard, is part of a growing body of sophisticated models of organs engineered to study processes within the human body. The most cutting-edge combine synthetic elements with live tissue. The microdevices — called “organs-on-a-chip” — are built to mimic the function and structure of human organs like the heart and lungs. They can be used to study a range of biological processes, from drug delivery in certain parts of the body to disease progression.
“Gut” models are popular targets for development because of the applications for pharmaceutical development: “orally absorbed drugs, nutrients and things like that,” Abby Koppes says.
By adding neurons, the Koppeses’ research takes gut-on-a-chip modeling a step further. “Enteric neurons are critical in maintaining organ homeostasis within the small intestine, and their dysregulation is implicated in gastrointestinal disorders and neurodegenerative diseases,” the paper’s abstract reads. “The addition of neurons is critical for developing more complex and biomimetic organ chip devices, especially as they become the standard for studying developmental biology, drug delivery, and disease progression.”
The structure uses synthetic materials you might find in a household fish tank (“We make everything layer by layer and so it’s a combination of different thermoplastics and tape,” Ryan Koppes says.) to house live cell tissue — from rodents in this case, but the model can accommodate human neurons.


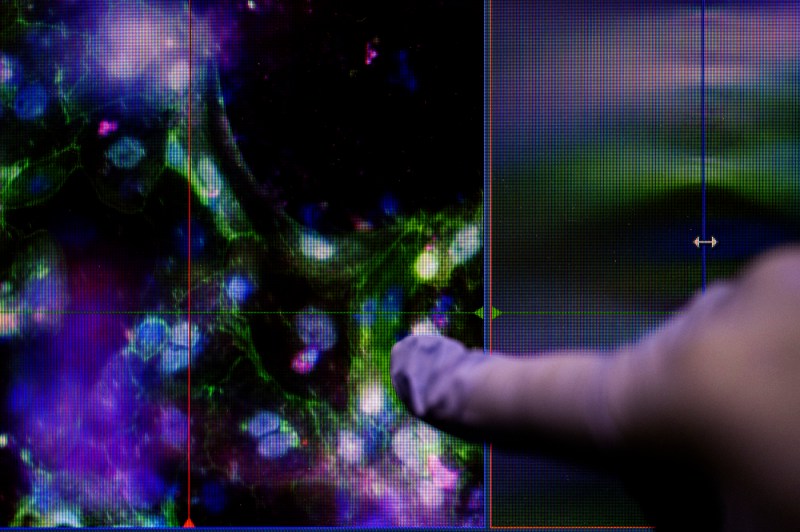

“In this paper we used cells that come from the intestine directly,” Abby Koppes says. “We isolate the epithelial cells, which are sort of like, you know, like cells on the outside of your skin protecting your body, but in your intestine you have the same thing. That system is used to protect your body from pathogens — interacts with your microbiota, food and everything. Those are the cells that we want to study because they’re really important for sensing the outside world and sending information kind of back into your body, and your nervous system in the gut is like right beneath that.”
“Including the nervous system is really the challenging aspect, because we know it can control how the gut functions,” she continues. “Without it, we see these models as really lacking.”
Abby Koppes’ research background revolves around tissue engineering and the nervous system; she began researching gut as a collaborator in Rebecca Carrier’s lab at Northeastern. Ryan Koppes’ team handles more of the technical aspects of the collaboration, adding instrumentation and engineering biomaterials. They’ve been working on this organ-on-a-chip model basically since Abby came to the Boston campus.
“This project was kind of initiated originally when the lab started in 2014,” she says. “It was something I talked about, in my ‘chalk talk’ trying to get the job here. So it’s been a dream over the past decade, really, of getting this to come to fruition.”
The Koppeses see the model as a starting point to build increasingly intricate models of the brain-gut connection, with a variety of applications.
“We’ve done all this work to build the system. Now we can start to manipulate,” Abby Koppes says. “We’re looking at Alzheimer’s and Parkinson’s as well, but we also have interest in things like environmental toxins and forever chemicals. We can apply the system to ask questions about how our bodies interact with our environment.”
Ryan Koppes thinks that because the system is small, and can be replicated efficiently with individual cells, there’s potential for drug therapies as well.
“As you start thinking about human disease, testing new compounds for toxicity and therapeutic screening efficacy is a huge selling point,” he says. “I think with these systems we can eventually make a patient-by-patient platform to really test different medications and disease pathways.”






