‘We don’t have any theory for this.’ Breakthrough discovery in materials science challenges current understanding of photoemission

What exactly is light—and what is it made of? It’s an age-old question that dates back to antiquity, and one of the most important investigations undertaken by scientists looking to understand the nature of reality.
The question of what comprises light—a form of energy that, as it bounces off of objects, allows us to see the world—has led to such spirited debate and discussion in the scientific community that it gave birth to a whole new field: quantum mechanics.
For many years, the debate about the nature of light was shaped by the mystery of its physical composition. Does light behave like a wave, or a particle? When Albert Einstein in the early 20th century proposed that light is both particulate in nature (containing small particles called photons) and wave-like, many were satisfied, if slightly uneasy, about his findings.

Einstein supported his new theory through his work on the so-called photoelectric effect, which earned him the Nobel Prize in Physics in 1921. First discovered by Heinrich Rudolf Hertz in 1887, the photoelectric effect describes the process by which light causes electrons to be ejected from a material when shone on it.
Now the leading experimental approach researchers use to probe the chemical and electronic properties of materials, photoemission has yielded practical applications for a range of technologies, specifically those that depend on light detection or electron-beam generation, like medical imaging devices and semiconductor manufacturing, among others.
But Northeastern researchers have made a discovery that challenges what scientists thought they knew about how photoemission is supposed to work, laying the groundwork for a new understanding of how light interacts with materials.
In a paper published in Nature on Wednesday, researchers observed what they described as the “unusual photoemission properties” of a particular material, strontium titanate—an oxide of the pair of chemical elements that first came into popular use more than half a century ago primarily as a diamond simulant.
Experimentally, the researchers used strontium titanate as a photocathode, or an engineered surface that can convert light into electrons through the photoelectric effect.
Photocathodes are also used in photodetector or sensory devices, such as photomultipliers; they are also used in infrared viewers, streak cameras, image intensifiers—or image amplifiers—and image converters.
Strontium titanate has historically been overlooked as a potential photocathode candidate, says Arun Bansil, university distinguished professor of physics at Northeastern, who co-authored the study.
“This material has many other uses and applications,” Bansil says.
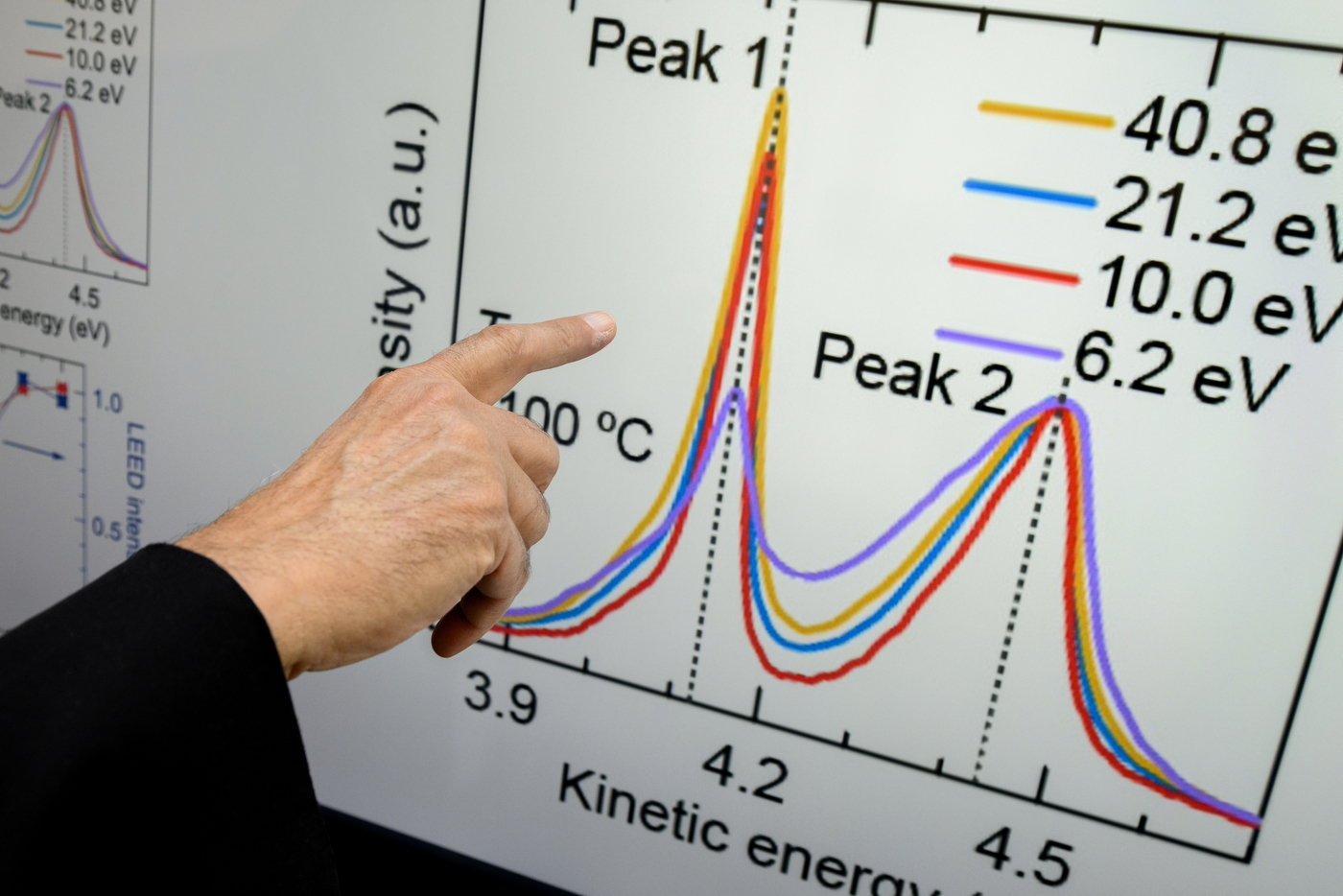
Using several photon energies in the 10 eV (electron-volt) range, researchers were able to produce a “very intense coherent secondary photoemission” stronger than anything seen before, Bansil says.
“This is a big deal because there is no mechanism within our existing understanding of photoemission that can produce such an effect,” Bansil says. “In other words, we don’t have any theory for this, currently, so it is a miraculous breakthrough in that sense.”
A secondary electron emission describes a phenomenon in which the primary electrons dislodged have suffered an energy loss as a result of collisions within the material prior to ejection.
“When you excite electrons, some of these electrons will actually come out of the solid,” Bansil says. “Primary electrons refer to those which have not scattered, whereas secondary electrons means they have undergone collisions before they’ve come out of the solid.”
The team of researchers, which included scientists from Westlake University in China, Lappeenranta-Lahti University of Technology (LUT) in Finland and Northeastern, said such a result points to “underlying novel processes” yet understood.
“The observed emergence of coherence in secondary photoemission points to the development of an underlying novel process on top of those encompassed in the current theoretical photoemission framework,” researchers wrote.
Bansil says the results upend what scientists thought they knew about the photoemission process, opening the door for new applications across industries that would harness the power of these sophisticated quantum materials.
“We all thought we understood the basic physics involved here, to the point where the development of applications is pursuant to a certain paradigm of theory and thought,” Bansil says. “As nature often does, this is where this paper throws a curveball at all of this.”
Experimental research was conducted in the laboratory of Ruihua He, an associate professor physics at Westlake University, with researchers Caiyun Hong, Wenjun Zou, Pengxu Ran, K. Tanaka, Changxi Zheng and Sheng Li. The theoretical team includes Bernardo Barbiellini, a physics professor at LUT University; Robert Markiewicz, a professor physics at Northeastern; Bansil; and graduate students Matt Matzelle and Wei-Chi Chiu of Northeastern’s physics department.
Tanner Stening is a Northeastern Global News reporter. Email him at t.stening@northeastern.edu. Follow him on Twitter @tstening90.