Drink that kombucha at your own risk: What a Northeastern scientist thinks about popular gut health advice
Type “gut health” in your search browser and you will find millions of popular web entries that tell you to take charge of your gut and eat specific foods to improve its condition.
First came probiotics. Then whole grains. Next, grocery stores started to devote whole display cases to kombucha. And now we are rediscovering the taste of other fermented foods.
So what is gut health and how can we support it?
“Gut health is one of these terms that are used sweepingly,” says Rebecca Carrier, professor of chemical engineering and principal investigator at Advanced Drug Delivery Research Lab at Northeastern University, who is studying the microbiome in order to develop gut models to facilitate further research.
For some, it can mean a healthy stomach. In medical literature, it might refer to the health of the lower gastrointestinal tract, which includes the small and the large intestines. And in a more holistic sense, gut health can be defined as effective digestion and absorption of food, absence of GI tract illnesses, sound immune status and overall state of well-being.


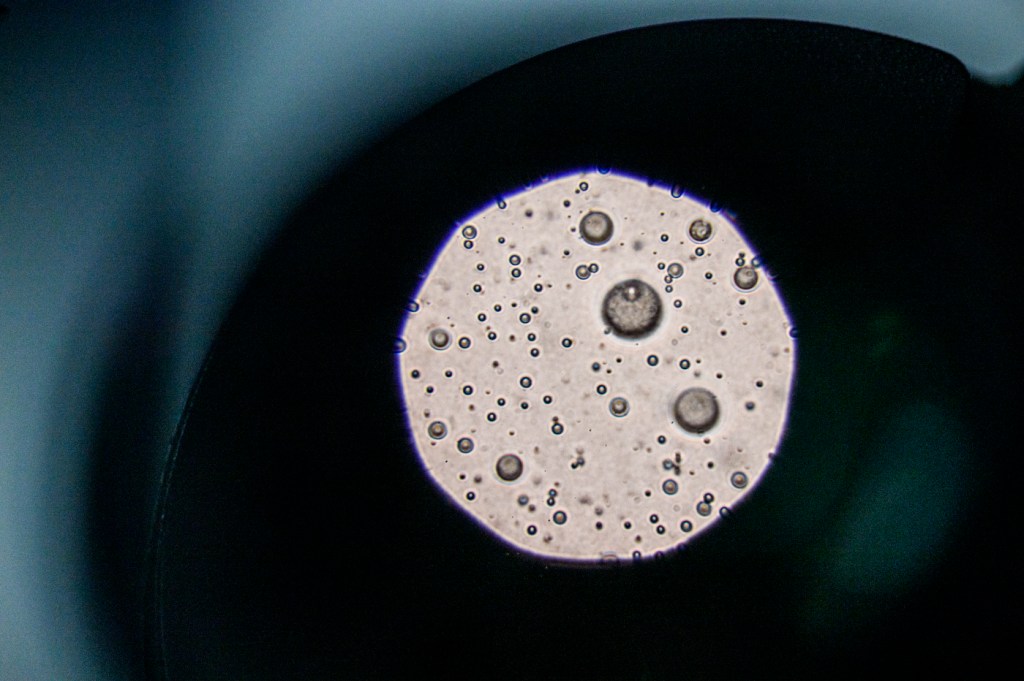
A few key components that determine gut health, Carrier says, are microbiome, or microorganisms that live in our gut; intestinal barrier permeability, which determines gut’s ability to contain undesirable contents; and the status of the immune cells in the gut.
There are more than 1,000 different bacteria species plus various strains that are present in every person’s gut, Carrier says. Some of them are core bacteria, which are common to certain populations of people, while others vary from person to person.
The microbiome is very important to our health because the bacteria feed off and break down the food that we consume into chemicals, beneficial for our bodies.
“There is more and more accumulating evidence that, at least with certain diseases, a shift in bacterial population leads to disease,” Carrier says.
The “good” bacteria in the gut prevent the overgrowth of the harmful bacteria by competing for nutrients and “territory,” or space on the mucous membranes of the gut.
Changes in the barrier properties and permeability of the gut are also associated with numerous diseases, Carrier says.
“Your barrier properties and your gut are keeping all these bacteria, all these other stimuli from food, which aren’t intended to just sort of freely pass through without being digested first,” she says. “If you change that barrier, you are going to get an immune response. And once you get that immune response, that can lead to all other sorts of problems.”
About 70% of our body’s immune cells are located in our gut underneath the epithelial barrier that lines the intestine. When these immune cells get activated, she says, it leads not only to intestinal inflammation, but they also send signals to everything else in the body.
When it comes to addressing gut health, doctors usually focus on treating already existing gut problems, Carrier says, such as inflammatory bowel disease like Crohn’s disease or celiac disease.
However, there is a lot of different, often confusing advice in the popular literature and on the internet when it comes to supporting gut health and preventing illnesses.
“The science that is underlying all these claims are still emerging,” Carrier says.
The scientific approach is to advise somebody to do something for therapeutic purposes only after there is a lot of evidence and there have been controlled clinical studies conducted in people, she says. Then scientists and doctors can say that consuming something will result in a specific outcome.
Carrier admits that there are a lot of traditional approaches to improving gut health that sometimes have similar beneficial effects as Western medicine that uses compounds synthesized and tested in people. The scientific community generally backs the idea that probiotics, prebiotics and fermented foods are beneficial to our health, she says.
Probiotics are live microorganisms that are beneficial to our bodies, and by consuming them we are typically trying to replace some of the bacteria populations associated with disease. There are probiotics on the market that contain a single bacterial species, and others that use a number of different bacteria.
“What fraction of those bacteria actually integrate into the gut microbiome? Does it happen in the upper GI tract or in the lower GI tract? How long does that last?” Carrier says. “We don’t really have a good hold on that.”
Scientists know that certain foods change gut permeability, she says, for example, certain fibers help bacteria produce short chain fatty acids that are the main source of nutrition for the cells in our colon, have anti-inflammatory properties and may reduce the risk of type 2 diabetes, obesity, heart disease, and other conditions.
“That’s what we call prebiotics,” Carrier says. “Something that’s feeding the good bacteria and allowing them to produce the things that they’re going to produce.”
Fermented foods like sauerkraut, yogurt, kefir, kombucha and kimchi contain both the bacteria themselves and the substances that the bacteria produce during the fermenting process.
The good news is that there have not been a lot of reported adverse events from eating probiotics, fiber or fermented food, Carrier says. From the risk versus benefit ratio standpoint, taking probiotics, eating a fiber-rich diet and fermented foods makes sense, she says.
“Some people will report with certain probiotics, for example, discomfort or bloating,” she says. “Okay, so then maybe that one in particular is not working for you.”
It is worthwhile to monitor whether consumption of “gut-healthy” foods affects you in a positive or a negative way, she says. Be careful of products that affect and can damage gut lining permeability like alcohol or anti-inflammatory drugs and avoid foods that you personally might be sensitive to like dairy and gluten.
Carrier also advises to do your research and look up the scientific studies that popular media are referencing in their articles.
Is it a significant number of scientific studies? Is it just a one-off study from one lab? Is it a reputable journal it was published in? These are the types of questions she would ask herself.
Although the scientists don’t quite know yet how to use certain approaches rationally and with confidence that a specific person will have a specific result, Carrier says, one thing is clear—our gut health is what we feed our bacteria for them to produce beneficial effects in our bodies and we can choose to be thoughtful about that.
Alena Kuzub is a Northeastern Global News reporter. Email her at a.kuzub@northeastern.edu. Follow her on Twitter @AlenaKuzub.






