This nanomaterial could be our best tool to make renewable energy cheaper

Nature isn’t always generous with its secrets. That’s why some researchers look into unusual places for solutions to our toughest challenges, from powerful antibiotics hiding in the guts of tiny worms, to swift robots inspired by bats.
Now, Northeastern researchers have taken to the trees to look for ways to make new sustainable materials from abundant natural resources—specifically, within the chemical structure of microfibers that make up wood.
A team led by Hongli (Julie) Zhu, an assistant professor of mechanical and industrial engineering at Northeastern, is using unique nanomaterials derived from cellulose to improve the large and expensive kind of batteries needed to store renewable energy harnessed from sources such as sunlight and the wind.
Cellulose, the most abundant natural polymer on Earth, is also the most important structural component of plants. It contains important molecular structures to improve batteries, reduce plastic pollution, and power the sort of electrical grids that could support entire communities with renewable energy, Zhu says.
“We try to use polymers from wood, from bark, from seeds, from flowers, bacteria, green tea—from these kinds of plants to replace plastic,” Zhu says.


One of the main challenges in storing energy from the sun, wind, and other types of renewables is that variation in factors such as the weather lead to inconsistent sources of power.
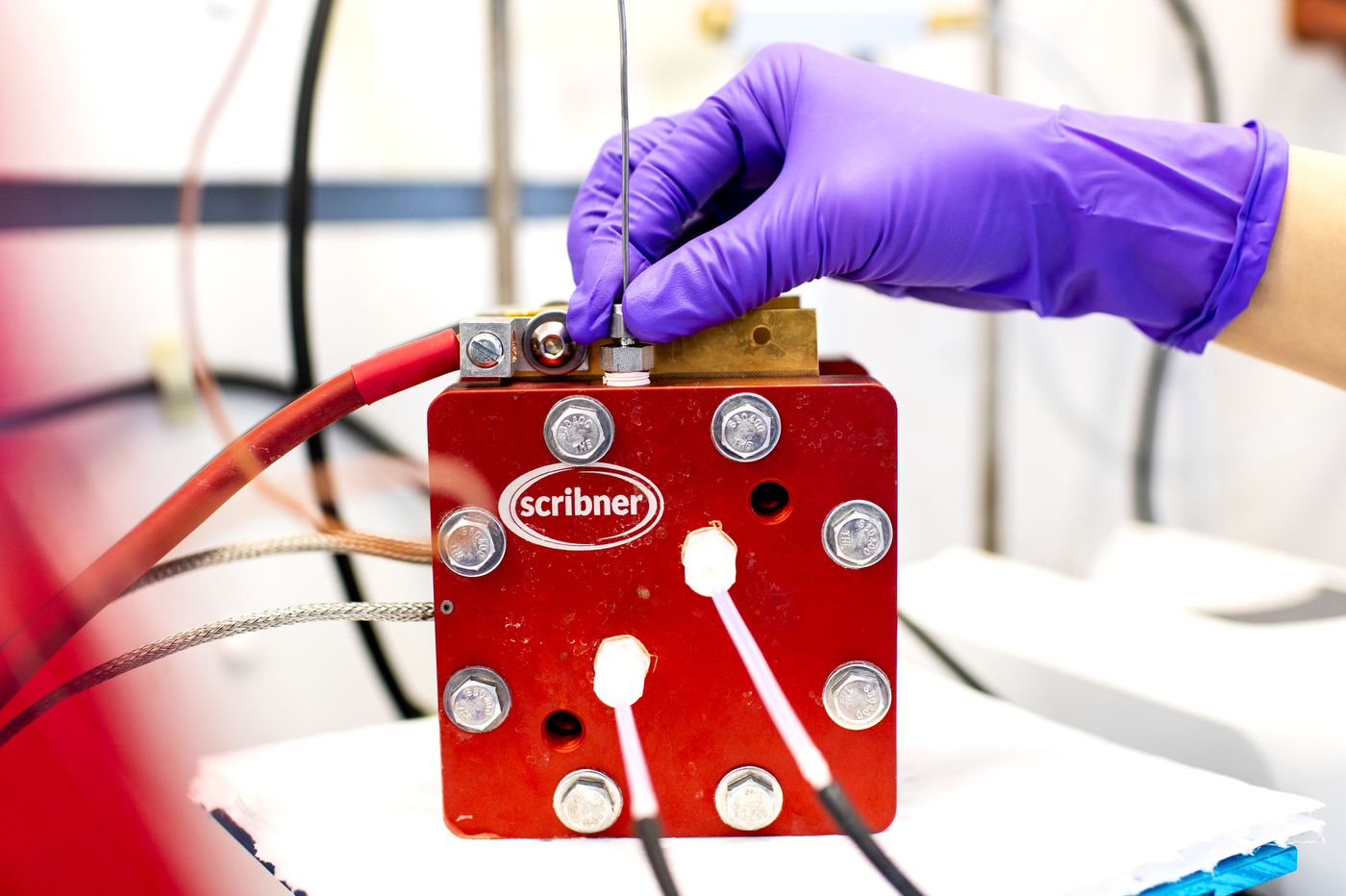
That’s where batteries with large capacity come in. But storing the large amounts of energy that sunlight and the wind are able to provide requires a special kind of device.
The most advanced batteries to do that are called flow batteries, and are made with vanadium ions dissolved in acid in two separate tanks—one with a substance of negatively charged ions, and one with positive ones. The two solutions are continuously pumped from the tank into a cell, which functions like an engine for the battery.
These substances are always separated by a special membrane that ensures that they exchange positive hydrogen ions without flowing into each other. That selective exchange of ions is the basis for the ability of the battery to charge and discharge energy.

Flow batteries are ideal devices in which to store solar and wind energy because they can be tweaked to increase the amount of energy stored without compromising the amount of energy that can be generated. The bigger the tanks, the more energy the battery can store from non-polluting and practically inexhaustible resources.
But manufacturing them requires several moving pieces of hardware. As the membrane separating the two flowing substances decays, it can cause the vanadium ions from the solution to mix. That crossover reduces the stability of a battery, along with its capacity to store energy.
Zhu says the limited efficiency of that membrane, combined with its high cost, are the main factors keeping flow batteries from being widely used in large-scale grids.
In a recent paper, Zhu reported that a new membrane made with cellulose nanocrystals demonstrates superior efficiency compared to other membranes used commonly in the market. The team tested different membranes made from cellulose nanocrystals to make flow batteries cheaper.
“The cost of our membrane per square meter is 147.68 US dollars, ” Zhu says, adding that her calculations do not include costs associated with marketing. “The price quote for the commercialized Nafion membrane is $1,321 per square meter.”



Their tests also showed that the membranes, made with support from the Rogers Corporation and its Innovation Center at Northeastern’s Kostas Research Institute, can offer substantially longer battery lifetimes than other membranes.
Zhu’s naturally derived membrane is especially efficient because its cellular structure contains thousands of hydroxyl groups, which involve bonds of hydrogen and oxygen that make it easy for water to be transported in plants and trees.
In flow batteries, that molecular makeup speeds the transport of protons as they flow through the membrane.
The membrane also consists of another polymer known as poly(vinylidene fluoride-hexafluoropropylene), which prevents the negatively and positively charged acids from mixing with each other.
“For these materials, one of the challenges is that it is difficult to find a polymer that is proton conductive and that is also a material that is very stable in the flowing acid,” Zhu says.
Because these materials are practically everywhere, membranes made with it can be easily put together at large scales needed for complex power grids.
Unlike other expensive artificial materials that need to be concocted in a lab, cellulose can be extracted from natural sources including algae, solid waste, and bacteria.
“A lot of material in nature is a composite, and if we disintegrate its components, we can use it to extract cellulose,” Zhu says. “Like waste from our yard, and a lot of solid waste that we don’t always know what to do with.”
For media inquiries, please contact Marirose Sartoretto at m.sartoretto@northeastern.edu or 617-373-5718.





