Northeastern University researchers use nanoparticles made by bacteria to fight antibiotic-resistant infections like MRSA

An estimated 700,000 people worldwide die from antibiotic-resistant infections every year, including strains of E. coli, Staphylococcus, and pneumonia. And if current trends continue, some predictions say the annual death toll could rise to 10 million by 2050, surpassing the amount of people killed by all cancers combined.
Microscopic particles, manufactured by those very same antibiotic-resistant bacteria, could replace traditional antibiotics and provide a solution to this looming crisis, says Thomas Webster, a professor of chemical engineering at Northeastern.
Webster and his colleagues are using bacteria to produce nanoparticles, metallic particles that are between one and 100 nanometers wide. (A single hair is about 80,000 nanometers wide.) The researchers have found that these nanoparticles are particularly effective at killing whatever type of cell was used to create them, including strains of bacteria that are resistant to traditional antibiotics.
“These particles that are made by living organisms are actually better than those that are made through synthetic chemistry,” says Webster, who is also the Art Zafiropoulo Chair in Engineering at Northeastern. “They can selectively target the bacteria or cell that made them.”
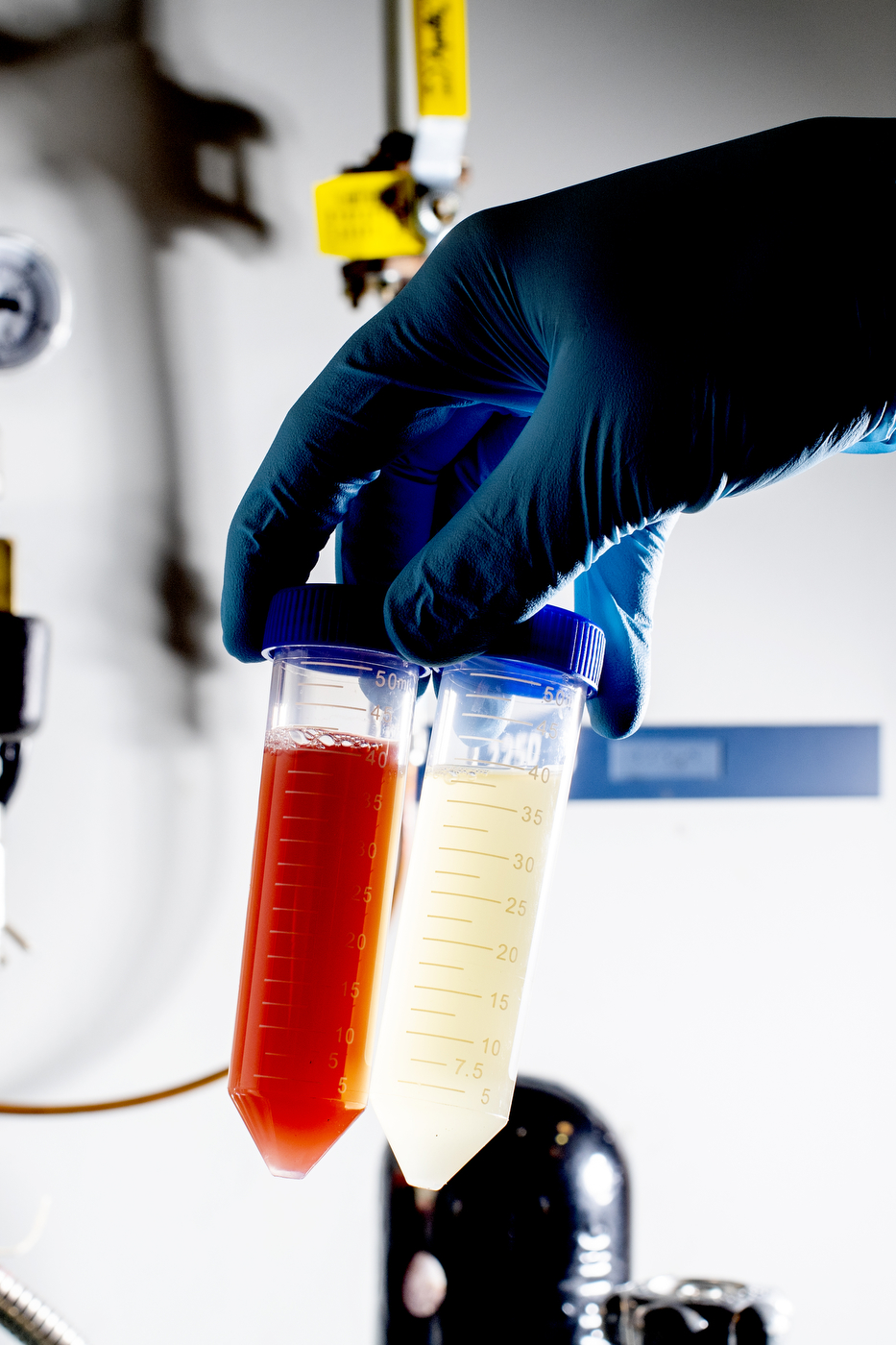

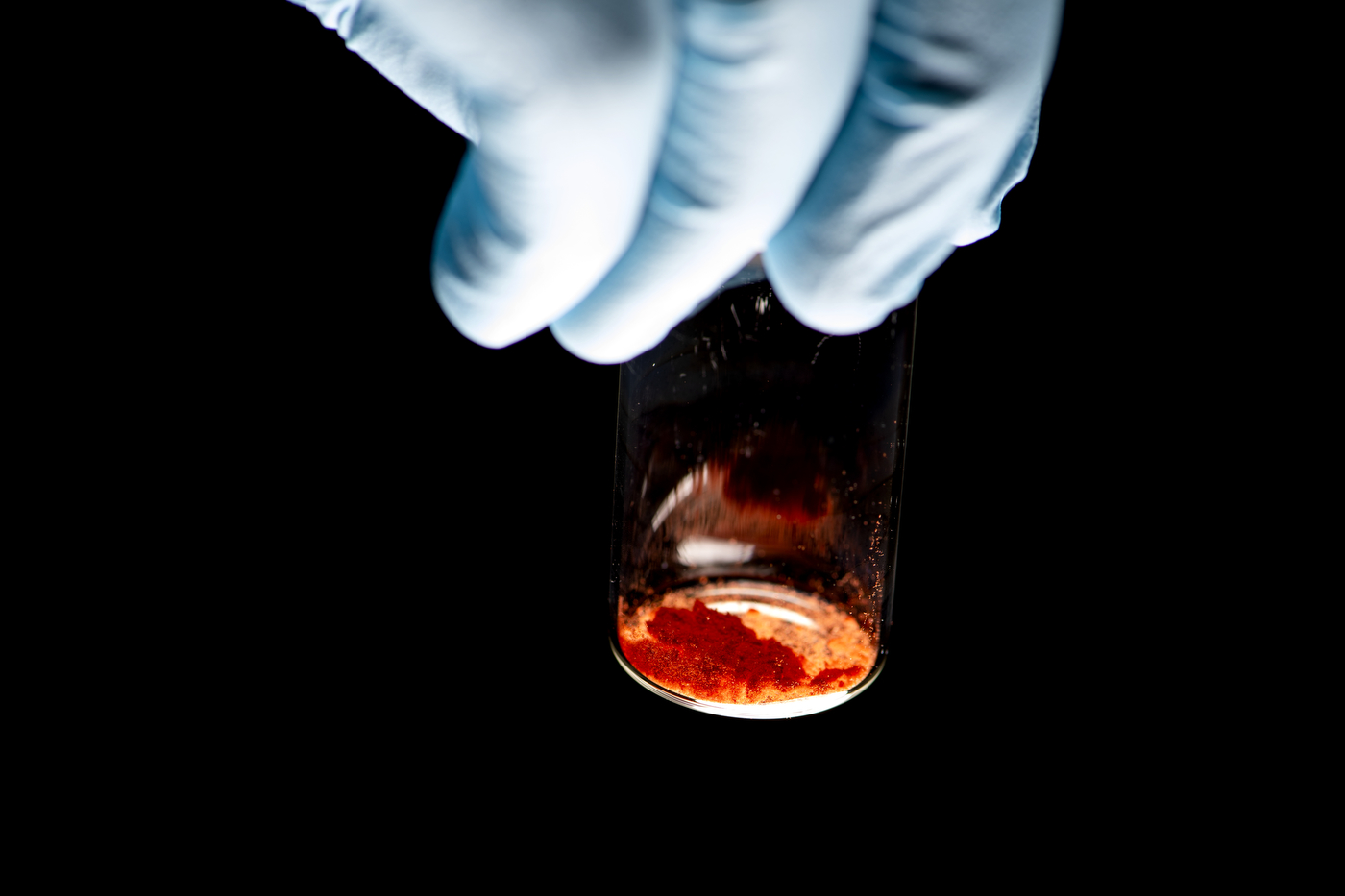
Because of their small size, nanoparticles can destroy cells by smothering them from the outside or disrupting cellular functions from the inside. The hard part is making sure that nanoparticles only kill the cells they are supposed to.
“We have a lot of very good bacteria in our body,” Webster says. “You just want to kill the harmful ones.”
Nanoparticles are typically produced synthetically, using chemical reactions. But by feeding bacteria or other cells the right chemical compound, the researchers have been able to use a cell’s internal mechanisms to synthesize nanoparticles instead.
While the researchers aren’t certain why these nanoparticles specifically target their creators, David Medina, a doctoral student in Webster’s lab, says the bacteria may be misidentifying the nanoparticles as “friendly.”

Thomas Webster, a professor of chemical engineering and the Art Zafiropoulo Chair in Engineering at Northeastern, wants to use nanoparticles to stop antibiotic-resistant bacteria. Photo by Adam Glanzman/Northeastern University
Bacterial cells are able to recognize one another. They may act to fight off something that registers as foreign, but they can coexist and cooperate with their own kind. When bacteria make nanoparticles, they coat them in a thin halo of protein. That protein layer may be making other bacteria of the same species flag them as “friendly” and let the nanoparticles get close.
“The natural coating comes from the bacteria,” Medina says. “They pull it inside, thinking that it’s something similar to them. But then, they find a surprise.”
Because of this deception, Medina refers to the nanoparticles as “nanometric Trojan Horses.” And, as often happens in science, the discovery was a happy accident.
Webster’s lab has been working with nanoparticles for two decades, he says, but like most researchers, they were making those nanoparticles through synthetic chemistry.
“Sometimes in that process, you have to use fairly toxic materials,” Webster says. When Medina began his doctoral research in the lab, he wanted to focus on more environmentally-friendly methods. “Through David’s efforts, we’re one of the few labs really pioneering this area that we call green nanomedicine, where you use either environmentally safe materials or living organisms to make nanoparticles.”
Nanoparticles have many potential uses in medicine and other fields, but Medina decided to see if his would be able to kill bacteria. He began growing colonies of cells, mixing in metallic salts for them to process, and then purifying the results into nanoparticles. Then he would mix those nanoparticles with a different species of bacteria, to see if the nanoparticles could kill them.
One day, he made a mistake.
“I was really tired,” Medina says. “I was preparing the experiment, and instead of mixing the nanoparticles with a different bacteria, I mixed them with the same.”
The nanoparticles, which had almost no effect on the bacteria he intended to test them on, effectively killed the bacteria that created them.
“David discovered that if we program MRSA [an antibiotic-resistant Staphylococcus bacteria] to make a nanoparticle, that nanoparticle can actually selectively kill MRSA,” Webster says. “And we’re seeing that across the whole spectrum. If you took a breast cancer cell and you programmed that cell to make a nanoparticle, that nanoparticle is more selective at killing breast cancer cells than the healthy cells that are in your body. That was totally unexpected.”
This discovery has become the center of Medina’s doctoral thesis, and could provide a way to combat the rising number of antibiotic-resistant infections. The researchers are also looking into using these methods to design nanoparticle-based cancer treatments.
“All we expected to do in this effort was to reduce the number of toxic chemicals that we use to make nanoparticles,” Webster says. “But we ended up discovering a whole family of nanoparticles that are actually more effective at doing what we want them to do: to kill bacteria, or kill tumor cells.”
For media inquiries, please contact media@northeastern.edu.





