Published on
New Northeastern lab plumbs the mysteries of the ticks and bacteria that cause Lyme
Constantin Takacs of Northeastern loves to study black-legged deer ticks and Borrelia burgdorferi, which is good news for everybody else
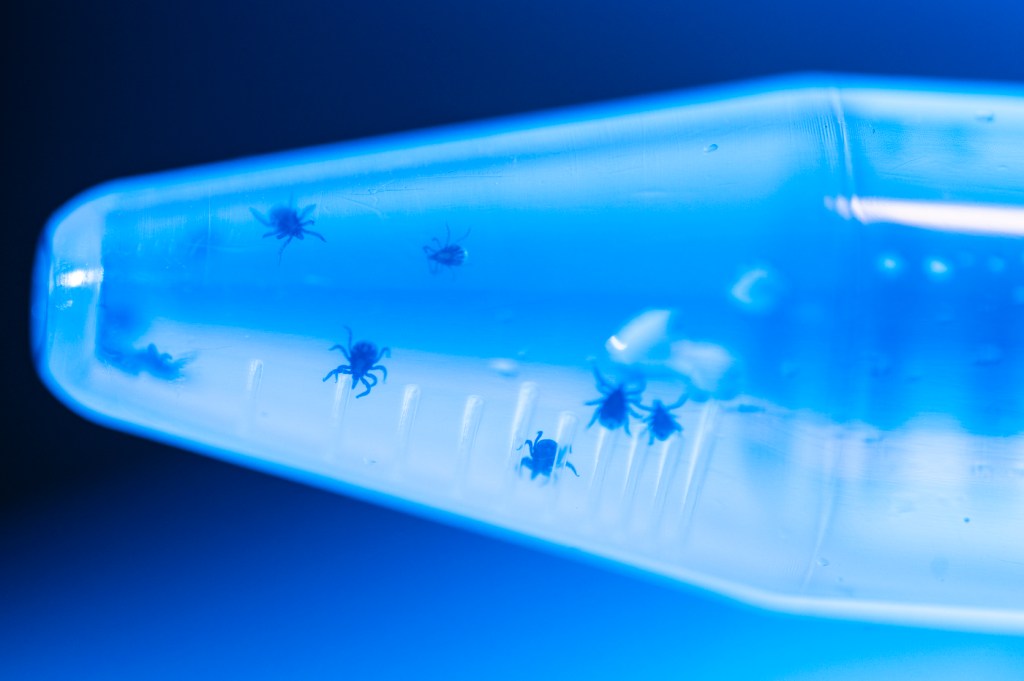
A sign at the entrance to the newly established laboratory of Northeastern University assistant biology professor Constantin Takacs warns visitors of the tiny menaces that dwell within.
“There are ticks present in the space,” it says. “Do not enter this room without the knowledge and permission of the Takacs lab.”
To make sure the poppyseed-sized black-legged ticks recently arrived from a breeding facility don’t escape, the doorway to the testing room is lined with a white double-sided sticky mat.
The precautions are meant to ensure researchers’ safety as they use new techniques to follow Borrelia burgdorferi, the bacterium that causes Lyme disease, inside the tick over the course of the tick’s two-year life cycle.
With cases of Lyme disease spreading and outpacing mosquito-borne illnesses in the U.S., it’s more important than ever to explore the cycle of disease and potential ways to interrupt it, Takacs says.
The little-understood tick
“We need to understand how the (Lyme disease bacteria) function and how they’re transmitted by ticks. And, therefore, we need to understand the tick itself,” he says.
Surprisingly little is known about black-legged or deer ticks, despite them being a vector, or carrier, of Lyme, a disease for which 476,000 people are treated annually, according to the Centers for Disease Control and Prevention.
The vector biology field has been driven by mosquitoes. “In other parts of the world, the mosquito is far more important as a disease vector than ticks are,” Takacs says. “But here, right now, it’s ticks that transmit most of the vector-borne disease.”
Lyme disease is not only endemic in the heavily populated Northeast, mid-Atlantic and upper Midwest states, but the pathogen that causes it can stick around in the infected host for a long time.
“If you take a lab mouse and you infect it (with Borrelia burgdorferi) and you don’t treat it with an antibiotic, it’s infected for life,” Takacs says.
Deer ticks are also tough customers, able to live months in the lab without a meal and yet still transmit Lyme disease.
These are the sort of biological facts that intrigue Takacs but sound like the stuff of zombie horror movies to almost anyone who has ever taken a walk in the woods or fields where ticks dwell.
‘A lot of ticks will die for science’
A major challenge facing tick researchers is the small size of the creature, which like a spider has eight legs as an adult and so is considered an acarid rather than an insect like the mosquito.
The larvae of black-legged ticks, also known as deer ticks, resemble dust, Takacs says. The nymphs that they molt into are the size of a poppy seed, while adults are typically compared to sesame seeds.
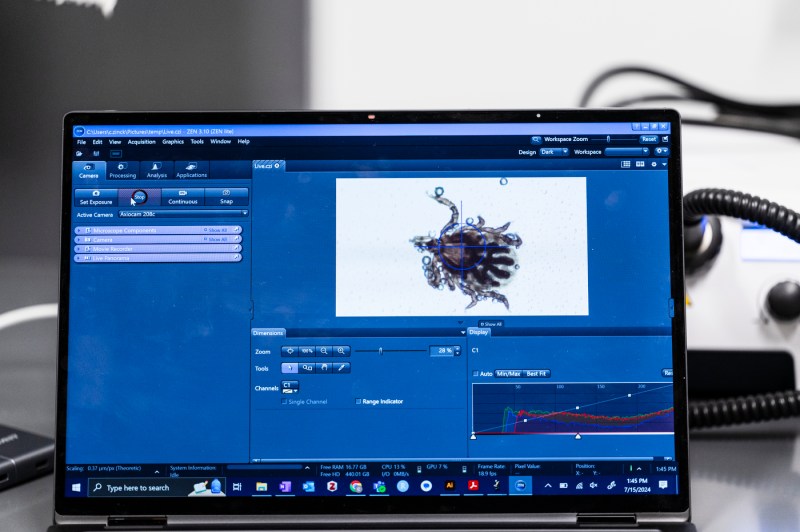

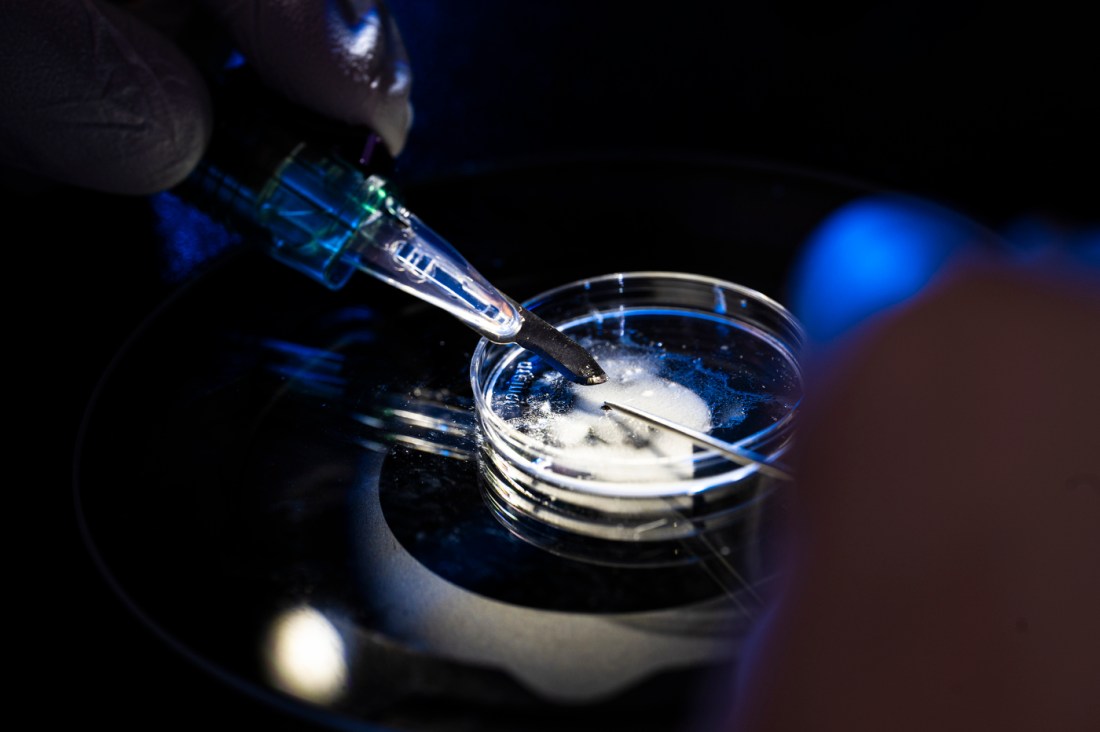
But tools new to the scientific community such as dissection stereo microscopes are giving Takacs and his researchers highly amplified views of nymphal ticks that arrived this summer from Oklahoma State University.
Using the microscope, which has a camera attached, postdoctoral research associate Chris Zinck shows a visitor how to look beyond the glint of a live tick’s hard outer body to see everything from its midgut to the hooks that anchor into their host victim’s skin.
Scientists want to know “What’s happening to the tick during its life cycle?” he says.
“A lot of that starts with what we can see,” says Zinck, who like Takacs wears a white lab coat and gloves instead of the traditional blue to better spot escaped ticks.
Zinck recently successfully dissected a nymphal tick after immobilizing it on a metal table chilled to 4 degrees Celsius. Ticks don’t like cold temperatures, so chilling them makes them easier to handle for dissection and other purposes.
“A lot of ticks will die for science,” Takacs says.
Blood meals
But first, the ticks will be fed a blood meal at every stage of their life, starting with larvae feeding on lab mice infected with Borrelia burgdorferi, Takacs says.
“In order to follow the bacterium inside a tick, we have to let the tick grow as it naturally does, so we have to feed the tick. And the only way we can feed the tick is by putting it on a mouse,” Takacs says.
In a literal feedback loop, the tick acquires the bacterium by feeding on an infected mouse, he says.
Featured Posts
Staining tick body parts with a special dye will allow researchers to see how the Lyme bacteria interacts with the tick’s anatomy as the pathogenic agent moves from gut to salivary gland and mouth and back again during and between feeding cycles, Takacs says.
Weird, and resilient, ticks
Once infected, the tick is able to transmit Lyme to mice, as well as other mammals including deer, dogs and humans.
One of the goals of the research is to disrupt tick anatomy or interactions to see whether that stops transmission of the bacteria, Takacs says.
It helps that scientists came up with a more complete iteration of the genome of the black-legged deer tick in early 2023, opening the way for the creation of genetic targets to reduce Lyme and other tick-borne diseases.
Takacs says his spirochete and vector biology lab at Northeastern will apply genetic modification techniques on ticks as well as on the bacteria that causes Lyme disease.
The twin-armed approach is aided by his training in both bacteriology and eukaryotic biology, which is the study of any cell with a clearly defined nucleus, including mice and ticks, Takacs says.
“I’m kind of straddling both worlds because I understand the bacterium, the microbe, but also the host. I like to study their interaction by looking at both angles,” he says.
Takacs, who came to Northeastern in January of 2023, says his fascination with Borrelia burgdorferi has only grown since his postdoctoral research days at Yale and Stanford.
Borrelia burgdorferi is a spiral-shaped bacterium called a spirochete. Another example of a spirochete is the one that causes syphilis, but borrelia is different enough to be labeled weird and almost certainly is unique in the world of bacteria.
In bad news for the body’s natural immune defenses, Borrelia burgdorferi has an “antigenic variation mechanism” that allows the bacteria to change a protein on their surface, Takacs says.
The surface protein is “akin to the armor of the organism,” he says. “It changes continuously. By the time the host immune system starts to attack the armor, the bacteria has changed the nature of the armor.”
This is different from the way most bacteria and pathogens enter the body and make a person feel ill.
“After a week or two, your immune system is going to kill the pathogen. Think about the last cold you had. Well, Borrelia burgdorferi is going to stay inside you for many months.”
In the case of the lab mouse, which can live one to five years, that’s for life, Takacs says.
Another way that the Lyme spirochete stands out from the rest of the bacterial crowd is by having more than 15 linear and circular plasmids in addition to one large linear chromosome.
Takacs says scientists theorize Borrelia burgdorferi evolved to have many genome segments so it could infect a variety of animal species.


Ticks on dinosaurs and Lyme in a mummy
Black-legged ticks are also resilient, almost shockingly.
“You can take a tick and put it in a jar at room temperature on the shelf. And as long as it doesn’t get dry, a year later, it’s still alive,” Takacs says.
Not only that, “It still has the same number of spirochetes,” he says. “It’s still able to transmit Lyme disease.”
Perhaps it’s no surprise that ticks and the Lyme spirochete are so tough, seeing as they have been around for millenia.
Ticks have been discovered in the fossils of feathered dinosaurs, while evidence of Lyme disease was found in the 5,300-year-old mummified remains of Otzi “the Ice Man.”
Lyme can affect the joints, heart and brain
The most common symptoms of Lyme are fever, fatigue, joint pains and a rash, although not every sufferer gets the rash.
Untreated, the Lyme spirochete can get into skin, heart tissue, joints and the outermost layer of protective tissue surrounding the brain and spinal cord, Takacs says.
“If you think about the symptoms of Lyme disease, you get a match between where the bacteria go and where there’s pathology,” he says.
The CDC says people treated in the early stages of Lyme disease with appropriate antibiotics usually experience a full recovery although it estimates that 5% to 10% of Lyme patients have persistent symptoms after early treatment.
In the meantime, the Global Lyme Alliance says that as many as 2 million Americans could suffer post-treatment disability.
The search for better Lyme treatment has led Northeastern University Distinguished Professor of Biology Kim Lewis to develop an antibiotic treatment he says is more targeted to borrelia than broad spectrum antibiotics currently in use such as doxycycline.
Lewis says the antibiotic, Hygromycin A, could also mop up residual pathogens. It is currently in clinical trials in Australia.
Takacs believes potential solutions lie in understanding the complex interactions between Borrelia burgdorferi, black-legged ticks and host animals such as lab mice.
Black-legged deer ticks also transmit other but less common diseases than Lyme, including the bacterial illness anaplasmosis; babesiosis, which is transmitted by a parasite; and Powassan, which is a viral disease.
Tick habitat is expanding. But this is still “sort of an area of mystery,” he says.
Cynthia McCormick Hibbert is a Northeastern Global News reporter. Email her at c.hibbert@northeastern.edu or contact her on X/Twitter @HibbertCynthia.